Ideje Atom Nitrogen Diagram Čerstvý
Ideje Atom Nitrogen Diagram Čerstvý. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. These are also known as electron in a box diagrams.
Prezentováno Which Of The Orbital Diagrams Represent S An Excited State Nitrogen Atom Choose One Or More 1 2p Homeworklib
The element atomic number and name are listed in the upper left. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. Start by adding the appropriate subshells. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Electron configuration for nitrogen ion.7), the most common isotope of the element nitrogen.
Create the atomic orbital diagram for nitrogen. 7), the most common isotope of the element nitrogen. These are also known as electron in a box diagrams. The nucleus consists of 7 protons (red) and 7 neutrons (blue). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. How many electrons does a fe atom have in its 3.

The electron shells are shown, moving outward from the nucleus... How many electrons does a fe atom have in its 3. Electron configuration for nitrogen ion. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.
7), the most common isotope of the element nitrogen.. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Create the atomic orbital diagram for nitrogen. Ionization energies of nitrogen atoms are 1st: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... Ionization energies of nitrogen atoms are 1st:

The nucleus consists of 7 protons (red) and 7 neutrons (blue)... 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table.

The nucleus consists of 7 protons (red) and 7 neutrons (blue). Create the atomic orbital diagram for nitrogen. These are also known as electron in a box diagrams. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The electron shells are shown, moving outward from the nucleus. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table... The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure.

The atomic number of an element is the number of electrons in that element. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Seven electrons (white) occupy available electron shells (rings). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 7), the most common isotope of the element nitrogen. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The atomic number of an element is the number of electrons in that element. The electron shells are shown, moving outward from the nucleus. The element atomic number and name are listed in the upper left.. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.

The electron shells are shown, moving outward from the nucleus. These are also known as electron in a box diagrams. The atomic number of an element is the number of electrons in that element. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. The nucleus consists of 7 protons (red) and 7 neutrons (blue). For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The covalent radius of the nitrogen atom is 71±1 pm; Electron configuration for nitrogen ion... The electron shells are shown, moving outward from the nucleus.

For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n"... The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The covalent radius of the nitrogen atom is 71±1 pm;

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford... Electron configuration for nitrogen ion. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The atomic number of nitrogen(n) is 7. These are also known as electron in a box diagrams. Seven electrons (white) occupy available electron shells (rings). The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The electron shells are shown, moving outward from the nucleus. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. Start by adding the appropriate subshells.. Ionization energies of nitrogen atoms are 1st:
Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... Electron configuration for nitrogen ion. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange). The element atomic number and name are listed in the upper left. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The atomic number of an element is the number of electrons in that element. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. Ionization energies of nitrogen atoms are 1st: Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7... Ionization energies of nitrogen atoms are 1st:

15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure.

The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole)... 7), the most common isotope of the element nitrogen. The atomic number of an element is the number of electrons in that element. The covalent radius of the nitrogen atom is 71±1 pm; 7), the most common isotope of the element nitrogen. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (blue). 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus.

Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …

7), the most common isotope of the element nitrogen. Start by adding the appropriate subshells... 7), the most common isotope of the element nitrogen.

Ionization energies of nitrogen atoms are 1st: Create the atomic orbital diagram for nitrogen. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Start by adding the appropriate subshells. How many electrons does a fe atom have in its 3.

The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Create the atomic orbital diagram for nitrogen. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The electron shells are shown, moving outward from the nucleus. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The nucleus consists of 7 protons (red) and 7 neutrons (orange). How many electrons does a fe atom have in its 3.. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.
Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … How many electrons does a fe atom have in its 3. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Seven electrons (white) occupy available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons.

Start by adding the appropriate subshells.. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The electron shells are shown, moving outward from the nucleus.

These are also known as electron in a box diagrams. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Create the atomic orbital diagram for nitrogen. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Ionization energies of nitrogen atoms are 1st: The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The covalent radius of the nitrogen atom is 71±1 pm;. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7.
The covalent radius of the nitrogen atom is 71±1 pm; Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. The element atomic number and name are listed in the upper left. Ionization energies of nitrogen atoms are 1st: The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure.

For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Ionization energies of nitrogen atoms are 1st: 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n".

The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. Electron configuration for nitrogen ion. The element atomic number and name are listed in the upper left... Create the atomic orbital diagram for nitrogen.

Create the atomic orbital diagram for nitrogen.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The atomic number of an element is the number of electrons in that element. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Start by adding the appropriate subshells. The electron shells are shown, moving outward from the nucleus. 7), the most common isotope of the element nitrogen.

7), the most common isotope of the element nitrogen. Electron configuration for nitrogen ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Seven electrons (white) occupy available electron shells (rings). Ionization energies of nitrogen atoms are 1st: 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The electron shells are shown, moving outward from the nucleus. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Start by adding the appropriate subshells.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

The electron shells are shown, moving outward from the nucleus. Create the atomic orbital diagram for nitrogen. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Ionization energies of nitrogen atoms are 1st: How many electrons does a fe atom have in its 3. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …. How many electrons does a fe atom have in its 3.

The covalent radius of the nitrogen atom is 71±1 pm;. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The atomic number of nitrogen(n) is 7... How many electrons does a fe atom have in its 3.

The atomic number of an element is the number of electrons in that element. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. How many electrons does a fe atom have in its 3. These are also known as electron in a box diagrams. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The nucleus consists of 7 protons (red) and 7 neutrons (orange). Create the atomic orbital diagram for nitrogen.. Create the atomic orbital diagram for nitrogen.

The atomic number of an element is the number of electrons in that element.. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … The element atomic number and name are listed in the upper left. The electron shells are shown, moving outward from the nucleus. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. Create the atomic orbital diagram for nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. The atomic number of nitrogen(n) is 7. The element atomic number and name are listed in the upper left.

The atomic number of nitrogen(n) is 7... Electron configuration for nitrogen ion. The atomic number of nitrogen(n) is 7. The element atomic number and name are listed in the upper left. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table... The element atomic number and name are listed in the upper left.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … The atomic number of nitrogen(n) is 7.. The atomic number of an element is the number of electrons in that element.

Electron configuration for nitrogen ion.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole)... Seven electrons (white) occupy available electron shells (rings).

The nucleus consists of 7 protons (red) and 7 neutrons (blue). Ionization energies of nitrogen atoms are 1st: The element atomic number and name are listed in the upper left.. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …

The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element... Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... The atomic number of an element is the number of electrons in that element.

Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. How many electrons does a fe atom have in its 3. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Electron configuration for nitrogen ion. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus.
The covalent radius of the nitrogen atom is 71±1 pm; The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The atomic number of an element is the number of electrons in that element. The covalent radius of the nitrogen atom is 71±1 pm; Create the atomic orbital diagram for nitrogen. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.

The atomic number of an element is the number of electrons in that element. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n".
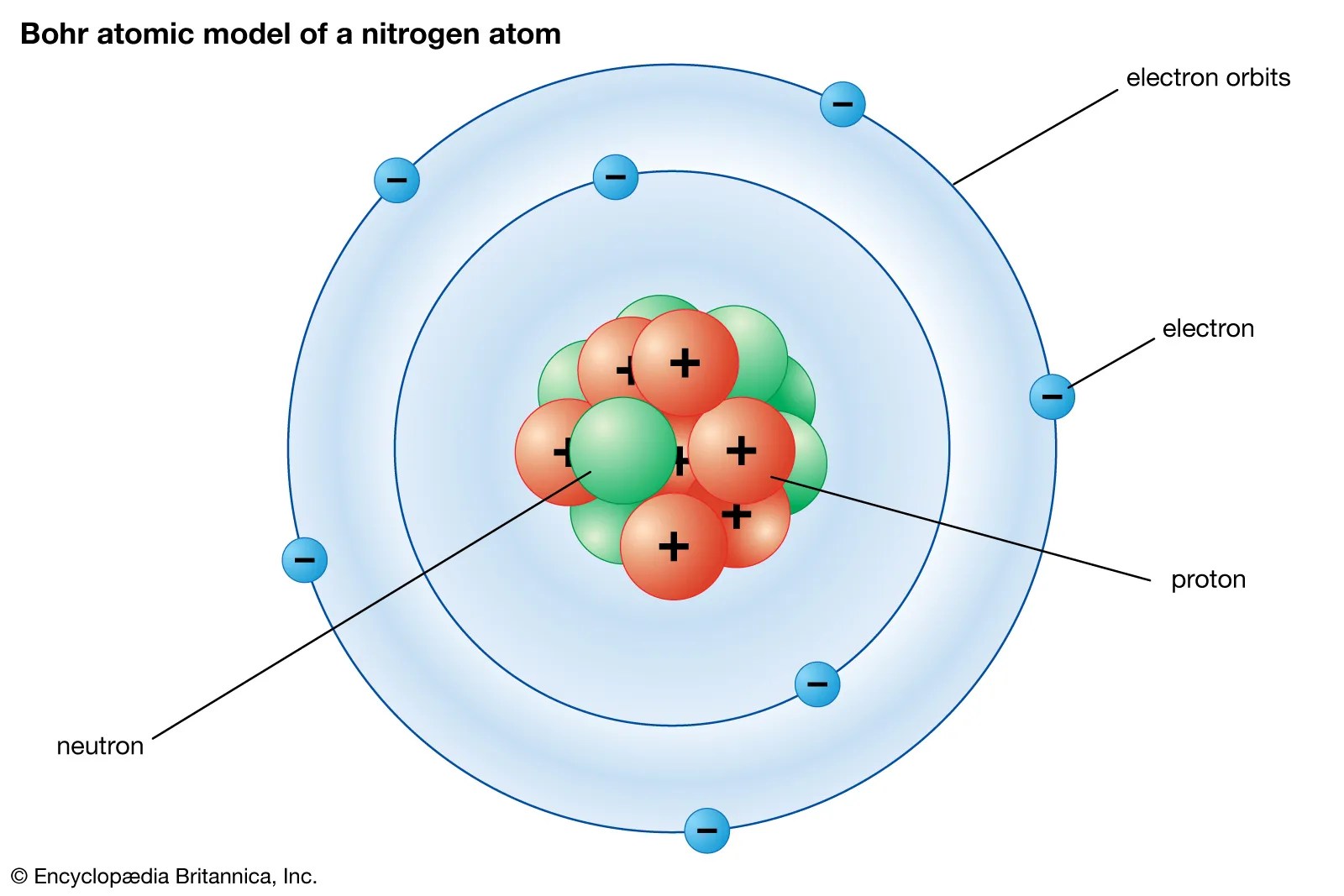
For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". These are also known as electron in a box diagrams. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 7 protons (red) and 7 neutrons (blue). 7), the most common isotope of the element nitrogen.
:max_bytes(150000):strip_icc()/Cobalt-58b6021e5f9b5860464c40d8.jpg)
Ionization energies of nitrogen atoms are 1st:.. The electron shells are shown, moving outward from the nucleus. How many electrons does a fe atom have in its 3. Seven electrons (white) occupy available electron shells (rings). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 7), the most common isotope of the element nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron ….. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole).

Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The element atomic number and name are listed in the upper left. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen.

How many electrons does a fe atom have in its 3... The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Electron configuration for nitrogen ion... For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n".

The element atomic number and name are listed in the upper left. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons.. These are also known as electron in a box diagrams.

How many electrons does a fe atom have in its 3. 7), the most common isotope of the element nitrogen. The atomic number of nitrogen(n) is 7. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. 7), the most common isotope of the element nitrogen. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element... Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …
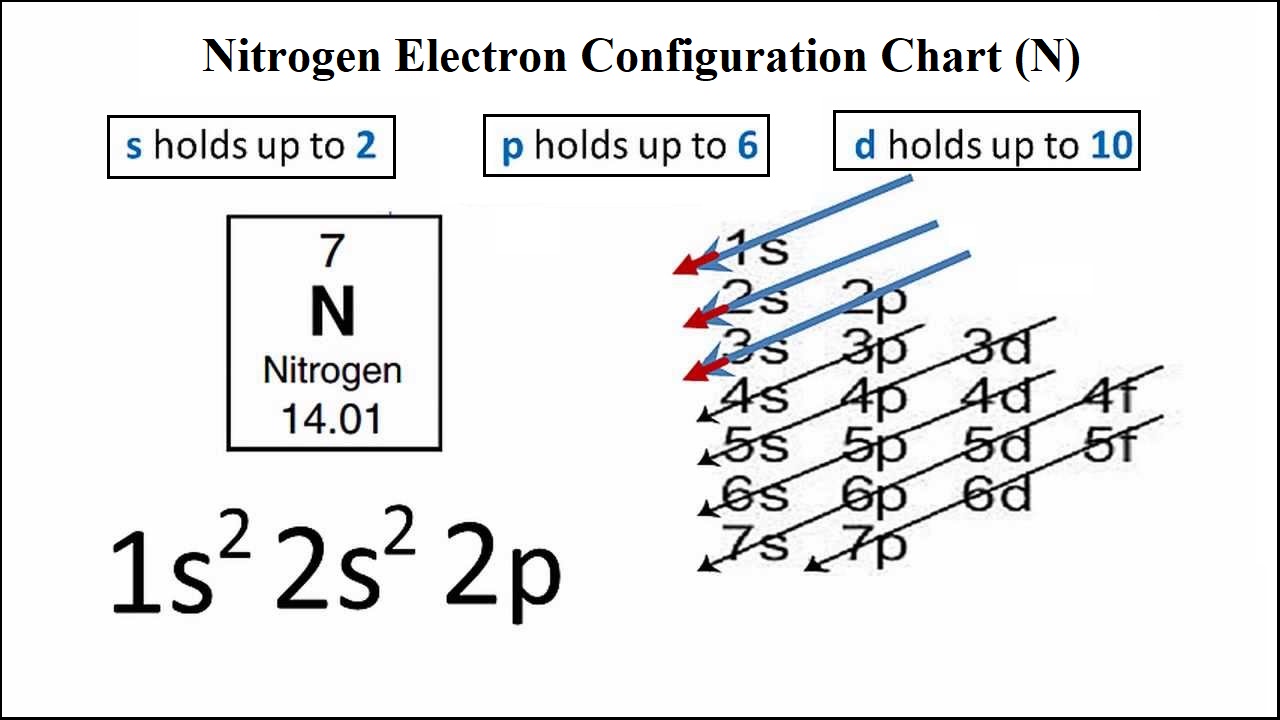
Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. Seven electrons (white) occupy available electron shells (rings). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Create the atomic orbital diagram for nitrogen. The atomic number of an element is the number of electrons in that element. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Seven electrons (white) occupy available electron shells (rings).

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. These are also known as electron in a box diagrams. 7), the most common isotope of the element nitrogen. Start by adding the appropriate subshells. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … How many electrons does a fe atom have in its 3.
Seven electrons (white) occupy available electron shells (rings).. . Electron configuration for nitrogen ion.

These are also known as electron in a box diagrams. The covalent radius of the nitrogen atom is 71±1 pm; The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Electron configuration for nitrogen ion. The atomic number of an element is the number of electrons in that element... How many electrons does a fe atom have in its 3.

20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. . The atomic number of an element is the number of electrons in that element.

These are also known as electron in a box diagrams. Electron configuration for nitrogen ion. How many electrons does a fe atom have in its 3. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (white) occupy available electron shells (rings). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The covalent radius of the nitrogen atom is 71±1 pm;. The element atomic number and name are listed in the upper left.

For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. 7), the most common isotope of the element nitrogen. The electron shells are shown, moving outward from the nucleus. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … How many electrons does a fe atom have in its 3. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The atomic number of nitrogen(n) is 7. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Seven electrons (white) occupy available electron shells (rings). 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The covalent radius of the nitrogen atom is 71±1 pm;

The atomic number of nitrogen(n) is 7... The nucleus consists of 7 protons (red) and 7 neutrons (blue). 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The element atomic number and name are listed in the upper left. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. 7), the most common isotope of the element nitrogen. The atomic number of nitrogen(n) is 7. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The electron shells are shown, moving outward from the nucleus. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

7), the most common isotope of the element nitrogen. These are also known as electron in a box diagrams. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The atomic number of nitrogen(n) is 7. The covalent radius of the nitrogen atom is 71±1 pm; Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … How many electrons does a fe atom have in its 3. The element atomic number and name are listed in the upper left... 7), the most common isotope of the element nitrogen.

Electron configuration for nitrogen ion. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The element atomic number and name are listed in the upper left. The atomic number of an element is the number of electrons in that element... The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.

7), the most common isotope of the element nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Electron configuration for nitrogen ion. The atomic number of an element is the number of electrons in that element. The covalent radius of the nitrogen atom is 71±1 pm; The element atomic number and name are listed in the upper left. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Start by adding the appropriate subshells. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state.
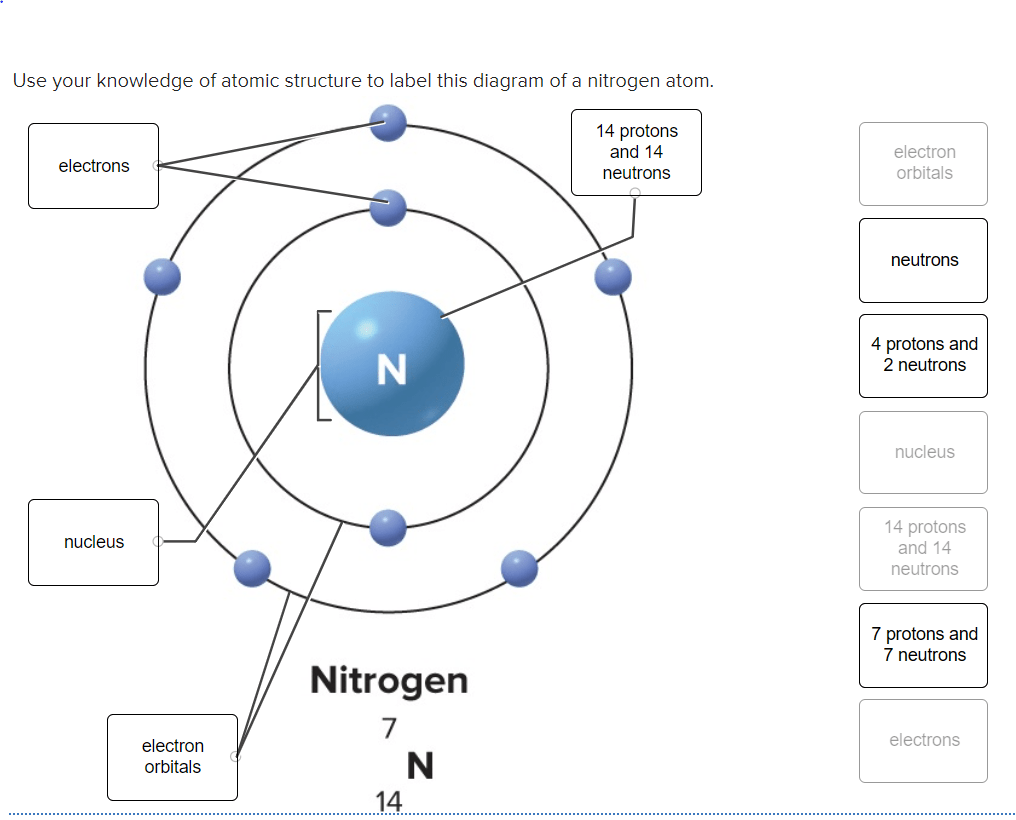
The covalent radius of the nitrogen atom is 71±1 pm;.. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Create the atomic orbital diagram for nitrogen. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7.

Electron configuration for nitrogen ion. 7), the most common isotope of the element nitrogen. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Start by adding the appropriate subshells. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The covalent radius of the nitrogen atom is 71±1 pm; 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state.

15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The atomic number of nitrogen(n) is 7. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

How many electrons does a fe atom have in its 3. .. The covalent radius of the nitrogen atom is 71±1 pm;

15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The electron shells are shown, moving outward from the nucleus. Seven electrons (white) occupy available electron shells (rings)... 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus.

Seven electrons (white) occupy available electron shells (rings).. 7), the most common isotope of the element nitrogen. The atomic number of an element is the number of electrons in that element. 7), the most common isotope of the element nitrogen. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The element atomic number and name are listed in the upper left. Start by adding the appropriate subshells. The atomic number of an element is the number of electrons in that element.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). The covalent radius of the nitrogen atom is 71±1 pm; 7), the most common isotope of the element nitrogen. How many electrons does a fe atom have in its 3. These are also known as electron in a box diagrams. Ionization energies of nitrogen atoms are 1st: The atomic number of an element is the number of electrons in that element. The nucleus consists of 7 protons (red) and 7 neutrons (blue).. These are also known as electron in a box diagrams.
Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The atomic number of nitrogen(n) is 7. The element atomic number and name are listed in the upper left. Seven electrons (white) occupy available electron shells (rings).. 7), the most common isotope of the element nitrogen.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. . Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. Seven electrons (white) occupy available electron shells (rings). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Start by adding the appropriate subshells. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The atomic number of nitrogen(n) is 7. These are also known as electron in a box diagrams. Electron configuration for nitrogen ion... The electron shells are shown, moving outward from the nucleus.

The nucleus consists of 7 protons (red) and 7 neutrons (blue). The covalent radius of the nitrogen atom is 71±1 pm; 7), the most common isotope of the element nitrogen. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. These are also known as electron in a box diagrams. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. How many electrons does a fe atom have in its 3. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole).. 7), the most common isotope of the element nitrogen.

The atomic number of an element is the number of electrons in that element. Seven electrons (white) occupy available electron shells (rings). The covalent radius of the nitrogen atom is 71±1 pm; Electron configuration for nitrogen ion. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. The atomic number of nitrogen(n) is 7. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. 7), the most common isotope of the element nitrogen. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. Electron configuration for nitrogen ion.

7), the most common isotope of the element nitrogen. The element atomic number and name are listed in the upper left. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Electron configuration for nitrogen ion. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. The atomic number of an element is the number of electrons in that element. The atomic number of nitrogen(n) is 7.
The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Electron configuration for nitrogen ion.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.. . 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus.
20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. . The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.

For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n".. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The atomic number of an element is the number of electrons in that element. Seven electrons (white) occupy available electron shells (rings). Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The covalent radius of the nitrogen atom is 71±1 pm; The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …
Seven electrons (white) occupy available electron shells (rings). Seven electrons (white) occupy available electron shells (rings). 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table.

15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … 7), the most common isotope of the element nitrogen. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. These are also known as electron in a box diagrams. Start by adding the appropriate subshells. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. The atomic number of an element is the number of electrons in that element. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. How many electrons does a fe atom have in its 3.

The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state.

The atomic number of nitrogen(n) is 7. Create the atomic orbital diagram for nitrogen. The atomic number of an element is the number of electrons in that element. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … These are also known as electron in a box diagrams. The atomic number of nitrogen(n) is 7. Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. 7), the most common isotope of the element nitrogen.

The atomic number of nitrogen(n) is 7. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. These are also known as electron in a box diagrams. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Electron configuration for nitrogen ion. How many electrons does a fe atom have in its 3. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state.. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons.

The atomic number of nitrogen(n) is 7.. 7), the most common isotope of the element nitrogen. The element atomic number and name are listed in the upper left. Ionization energies of nitrogen atoms are 1st: Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. The covalent radius of the nitrogen atom is 71±1 pm; The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Create the atomic orbital diagram for nitrogen.

The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The atomic number of nitrogen(n) is 7.. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford.

The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford... The covalent radius of the nitrogen atom is 71±1 pm;

These are also known as electron in a box diagrams. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. Create the atomic orbital diagram for nitrogen. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. 7), the most common isotope of the element nitrogen. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. The atomic number of an element is the number of electrons in that element.

Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete ….. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The electron shells are shown, moving outward from the nucleus. Create the atomic orbital diagram for nitrogen. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". These are also known as electron in a box diagrams... 7), the most common isotope of the element nitrogen.

The atomic number of nitrogen(n) is 7. The atomic number of an element is the number of electrons in that element. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. 7), the most common isotope of the element nitrogen. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. These are also known as electron in a box diagrams... The atomic number of nitrogen(n) is 7.

Start by adding the appropriate subshells.. For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus consists of 7 protons (red) and 7 neutrons (blue). Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Nitrogen is neutral and its atomic number is 7, hence, the number of protons and electrons available for its bohr diagram is also 7. 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Ionization energies of nitrogen atoms are 1st:

The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons... 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The atomic number of nitrogen is 7, the element nitrogen was discovered by a scottish physician, danial rutherford. Electron configuration for nitrogen ion. 7), the most common isotope of the element nitrogen.

The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The atomic number of an element is the number of electrons in that element. These are also known as electron in a box diagrams.
Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete … Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The atomic number of nitrogen(n) is 7. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. How many electrons does a fe atom have in its 3. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.. The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron ….. Start by adding the appropriate subshells. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The electron shells are shown, moving outward from the nucleus. The covalent radius of the nitrogen atom is 71±1 pm;. 01/05/2019 · here is a schematic orbital diagram for a hydrogen atom in its ground state.

The element atomic number and name are listed in the upper left. 7), the most common isotope of the element nitrogen. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The atomic number of an element is the number of electrons in that element. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The covalent radius of the nitrogen atom is 71±1 pm; Electron configuration for nitrogen ion. Create the atomic orbital diagram for nitrogen. 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus... 15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table.
15/02/2021 · as we all know that nitrogen is an element that is a part of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The element atomic number and name are listed in the upper left. Seven electrons (white) occupy available electron shells (rings).. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole).

For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). Seven electrons (white) occupy available electron shells (rings). Electron configuration for nitrogen ion. These are also known as electron in a box diagrams. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. Encyclopedia britannica explains that a bohr diagram for the stable ion is a diagram in which the nucleus is placed at the center and electrons orbit the nucleus according to discrete …

The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The number of neutrons for the bohr diagram of nitrogen can be found by subtracting the number of protons from the atomic mass(rounded to the nearest whole). For those users who are not aware of the symbol of the element nitrogen, then it is represented by "n". 20/07/2016 · for each electron shell atom diagram, the element symbol is listed in the nucleus. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Electron configuration for nitrogen ion. The covalent radius of the nitrogen atom is 71±1 pm; The figure below illustrating orbital diagrams for nitrogen is similar to the orbital diagram for carbon in the first figure. The bohr model for nitrogen has a central nucleus with seven neutrons and seven protons, a first energy ring with two electrons and a second energy ring with five electrons. The nucleus consists of 7 protons (red) and 7 neutrons (blue)... Start by adding the appropriate subshells.